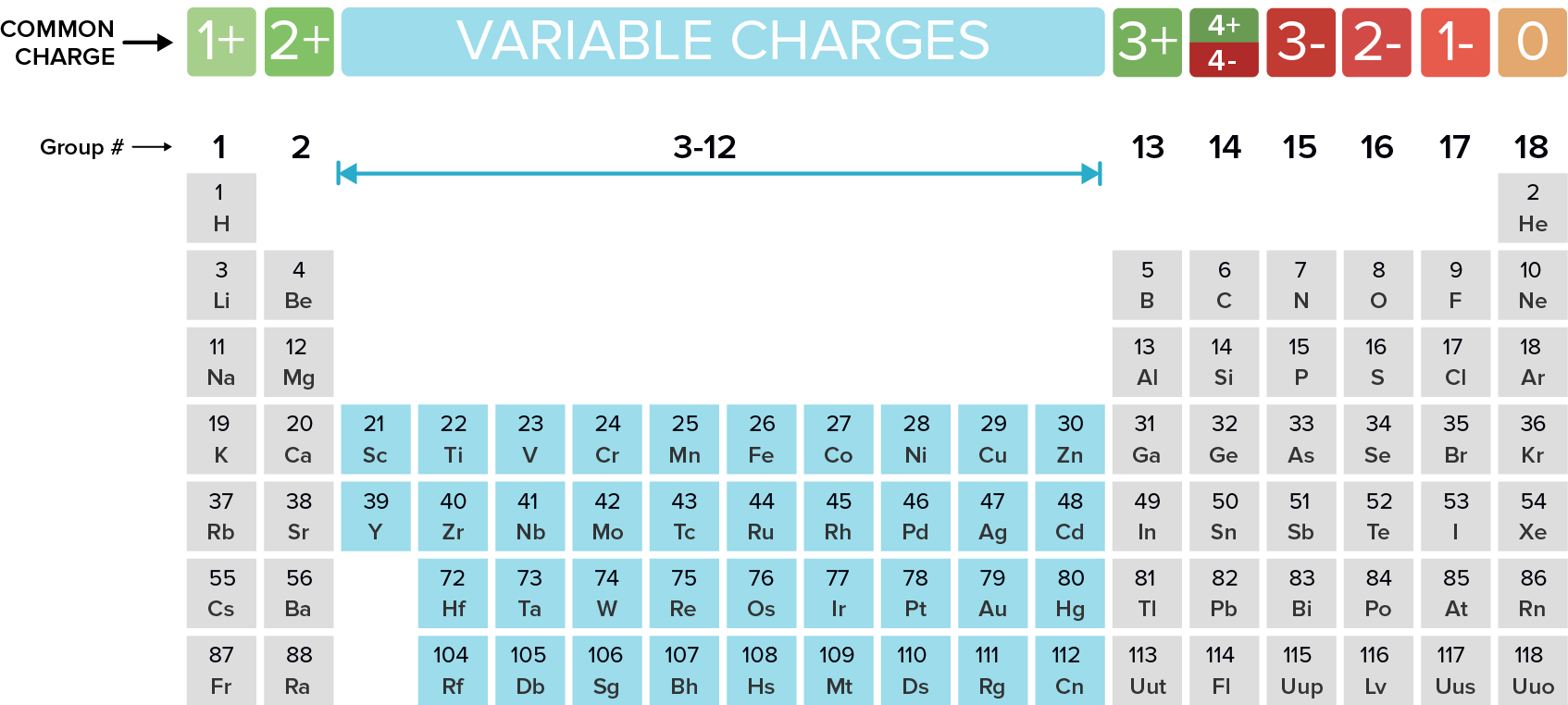
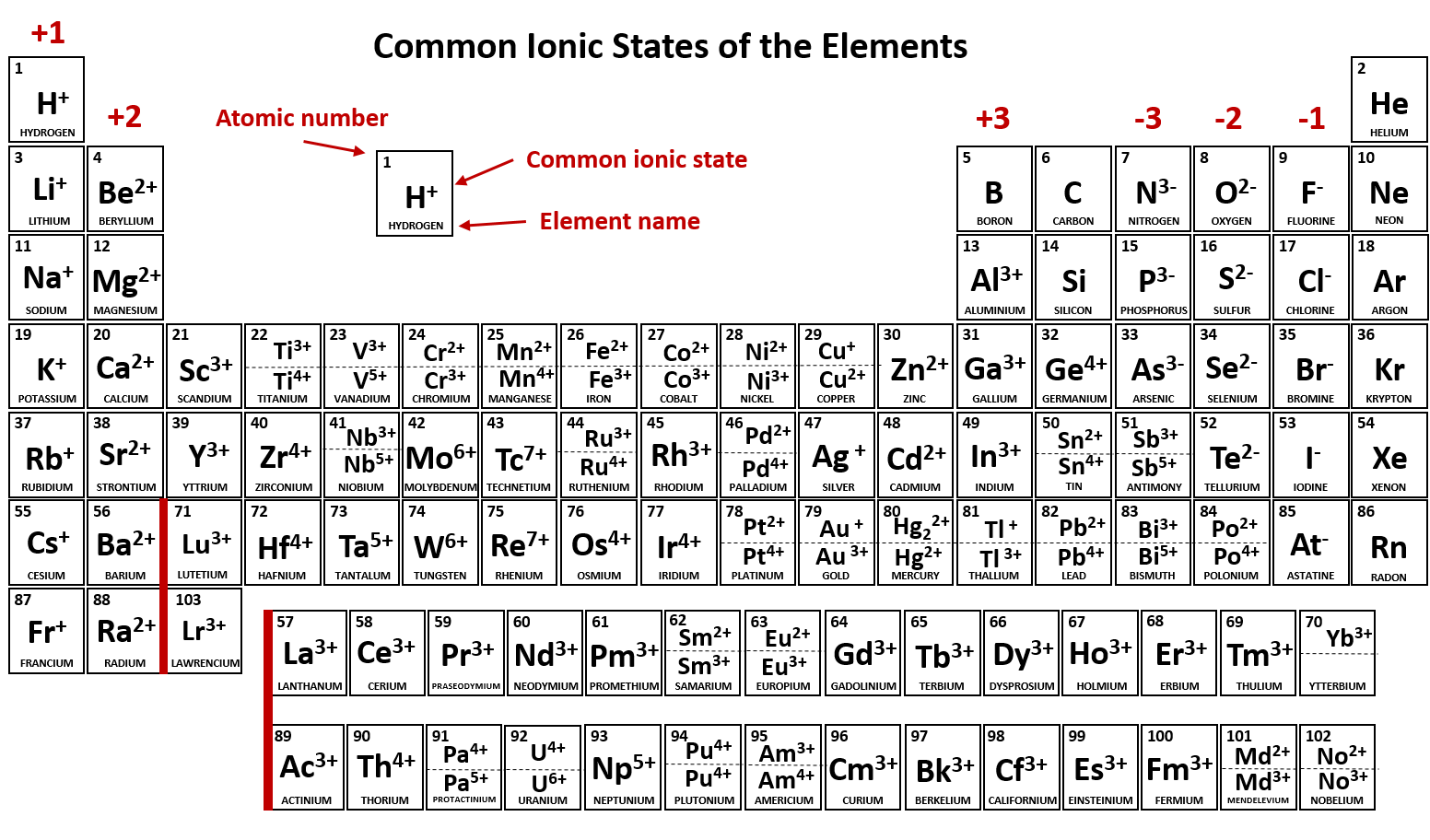
What Typically Forms Ions With a 2+ Charge
Here is the full list of metals in group two 2 charge. A Alkaline earth metals B Halogens C Chalcogens D Alkali metals E Transition metals Answer.
Naming Monatomic Ions And Ionic Compounds Article Khan Academy
D oxygen E nitrogen Answer.

. See the answer See the answer See the answer done loading _____ typically form ions with a 1- charge. Law of constant composition. If an element has 3 valence electrons what charge will likely form on its ion.
All elements in column 2 of the periodic table consistently form ions with a charge of two plus. Their atoms have 6 valence electrons and need 2 more to have a full valence shell of 8 electrons in order to become stable. The elements that are most likely to form 2- ions are the group 16 elements.
Which of the ions or atoms below have the same electronic configuration as Ar. Therefore B is the correct option. Group 1 metals commonly lose only one electron to form ions with a single positive charge.
_____ typically form ions with a 1- charge. Report an issue. If more than one unit of a polyatomic ion is needed place parentheses around the polyatomic ion.
Which of the following has a -3 charge. Group 2 elements are alkaline earth metals. Sodium forms an ion with a charge of _____ 1 aluminum forms an ion with a charge of.
A Alkaline earth metals B Halogens C Chalcogens D Alkali metals E Transition metals. When they gain 2 electrons in order to have 8 valence electrons an octet they gain a 2- charge. Chlorine typically forms an ion with a charge of -1.
This is a statement of _____ atoms are composed of protons neutrons and electrons. Which elements below are likely to form ions with the same charge as chlorine. On the other hand until finally 1986 the corporation accomplished among its primary aims.
Which statement best explains why hydrogen forms ions that do not follow the octet rule. B Alkaline earth metals. Breaking to the American market.
All the other elements in group 1 are alkali metals. What happens when an ionic compound dissolves in water. Alternatives 5 -3 -5.
The nonmetals in Group 16 gain two electrons to form ions with a 2 charge. This concept is explained in the following video. 7 energy levels and a Valence of 5 d.
This column is commonly referred to as. Because electrons have a charge of 1. A molecule of water contains hydrogen and oxygen in a 18 ratio by mass.
What group tends to form 2 ions. Lithium ion sodium ion potassium ion hydrogen ion silver ion ammonium ion copperI ion The element that forms a 2 plus ion with. They are the most reactive of all metals and along with the elements in group 17 the most reactive elements.
Here is the full list of metals in group one 1 charge. 3 energy levels and a Valence of 1. Identify the formulas and charges of the cation and anion.
A Alkaline earth metals B Halogens C Chalcogens D Alkali metals E Transition metals. 2 bromine form an ion with a charge of. 5 __________ typically form ions with a 2 charge.
3 energy levels and a Valence of 7 c. The noble gases do not normally gain or lose electrons and so do not normally form ions. The cations and anions separate and become surrounded by water molecules.
These metals contain 2 valence electrons so in order to acquire a noble gas configuration they will lose their 2 electrons and attain a 2 charge. Group 2 elements are alkaline earth metals. Place the cation first in the formula followed by the anion.
3 calcium forms an ion with a charge of. Alkaline earth metals B. The element in the Seventh family and Third period will have.
This means that it needs to gain two electrons to obey the octet rule and have a full outer shell of electrons eight. Elements in the alkali metals family will form ions with a charge of. Answer 1 of 7.
22 _____ typically form ions with a 2 charge. _____ typically form ions with a 2 charge. These metals contain 2 valence electrons so in order to acquire a noble gas configuration they will lose their 2 electrons and attain a 2 charge.
The ammonium ion contains one nitrogen atom _____ hydrogen atoms and has a charge of ______. This problem has been solved. Which group is most reactive.
Oxygen is in group six in the periodic table so it has six electrons in its valence shell. What Typically Form Ions With A 2 Charge What Typically Form Ions With A 2 Charge - In the course of the 1980s Hyundai observed rapid progress producing substantial inroads into global marketplaces. A 11 _____ typically form ions with a 2 charge.
What is NOT one of the postulates of Daltons atomic theory. Determine how many of each ion type is needed to make a neutral compound. 7 energy levels and a Valence of 3 b.
Typically forms ions with a 2 charge. Hydrogen and Alkali Metals It is the most common element in the universe. 2 Barium forms an ion with a charge of.
Answer choices 3 5-3-5 3.

5 1 Ionic And Molecular Compounds Introductory Chemistry

Pin By Ayodele Williams On My Saves Ionic Bonding Periodic Table Ionic
No comments for "What Typically Forms Ions With a 2+ Charge"
Post a Comment